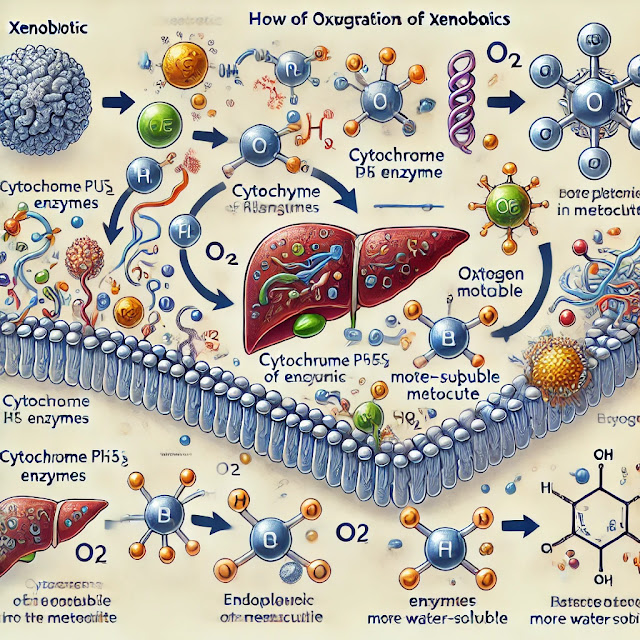
1. Cytochrome P450 Enzymes (CYP450)
The CYP450 enzymes are a superfamily of heme-containing enzymes located primarily in the liver but also found in other tissues. They play a central role in the oxidative metabolism of xenobiotics.
2. The Oxidation Process
The oxidation of xenobiotics typically involves two phases:
Phase I Reactions: Functionalization
In this phase, the xenobiotic undergoes structural modification, primarily through oxidation, which often introduces or exposes a functional group (e.g., -OH, -NH2, -SH, -COOH). The key steps in this process include:
- Substrate Binding: The xenobiotic (substrate) binds to the active site of the CYP450 enzyme.
- Electron Transfer: The enzyme-bound substrate undergoes one-electron reduction via electron transfer from NADPH (Nicotinamide Adenine Dinucleotide Phosphate) through a flavoprotein reductase.
- Oxygen Activation: Molecular oxygen (O2) binds to the reduced enzyme-substrate complex. One oxygen atom is incorporated into the substrate (forming a hydroxylated product), and the other oxygen atom is reduced to water.
- Product Release: The oxidized xenobiotic (now more hydrophilic) is released from the enzyme.
The overall reaction can be simplified as:
Phase II Reactions: Conjugation
After Phase I, the oxidized xenobiotic often undergoes Phase II reactions, where it is conjugated with endogenous substrates such as glucuronic acid, sulfate, glycine, or glutathione. These conjugation reactions further increase the water solubility of the xenobiotic, facilitating its excretion.
Types of Oxidation Reactions
Various types of oxidation reactions can occur, including:
- Hydroxylation: Addition of an -OH group, typically on aromatic rings or aliphatic chains.
- Epoxidation: Formation of an epoxide ring by adding an oxygen atom to a double bond.
- N-, O-, and S-dealkylation: Removal of alkyl groups from nitrogen, oxygen, or sulfur atoms.
- Deamination: Removal of an amino group.
- Sulfoxidation: Oxidation of sulfur-containing compounds to sulfoxides or sulfones.
Example: Hydroxylation by CYP450
An example of a hydroxylation reaction catalyzed by CYP450 is the conversion of benzene to phenol:
Importance and Implications
The oxidation of xenobiotics is a crucial detoxification mechanism that protects the body from potentially harmful compounds. However, it can also lead to the formation of reactive intermediates that may be toxic or carcinogenic. Understanding these mechanisms is vital for drug development, predicting drug interactions, and managing adverse drug reactions.
In summary, the oxidation of xenobiotics involves complex enzymatic processes, primarily mediated by cytochrome P450 enzymes, that modify and prepare foreign compounds for excretion from the body.








0 Comments
Thanks for your feedback, i'll get back to you soon